Hydrogen: the energy of the future?
Hydrogen could eventually replace oil. But only if we can meet the challenges of “greening” its production, making its use safe, and reducing the costs of this energy source.
An investigation by Pierre-Yves Bocquet - Published on , updated on
Hydrogen is exciting great hopes. It could quite simply spur a new industrial and energy revolution. All over the world, ambitious ‘hydrogen plans’ are signalling the transition from the era of total dependence on petrol, which is destined to come to an end, to a new decarbonized era. A world in which hydrogen would contribute to greening the footprint of the transportation sector and a great many other sectors of the economy. Some have been advocating this revolution for many years, but before it can succeed, numerous technical challenges must be overcome. How can hydrogen be produced with a smaller carbon footprint than is the case today? And at a cost that would reduce its price at the pump, which is currently much higher than that of gasoline? How can we make the most of its huge energy potential? How can it be transported and stored efficiently and safely? How can the gigantic infrastructure that will accompany this transition be deployed at a reasonable cost? The challenges are as enormous as the hopes raised by the smallest and lightest of all atoms.
A promising energy… but a long time coming
After many false starts, the climate emergency finally seems to be driving the transition to hydrogen.
Hydrogen has long been seen as a possible successor to fossil fuels, a substitute for the latter offering low greenhouse gas emissions. Whereas hydrocarbons emit many greenhouse gases, sometimes toxic, and fine particles that are dangerous to health, hydrogen combustion produces only water. The problem is that there are added costs associated with the transition to this so-called ‘decarbonized’ energy, which have held back its development until now. However, faced as we are with the urgent need to address climate change, several countries have announced ambitious plans to achieve carbon neutrality – by 2050 in Europe, and by 2060 in China, for example. Hydrogen is set to play a central role in this transition, provided it can be produced, transported and stored under acceptable environmental and economic conditions. And this is not a foregone conclusion. Various national plans announced in recent months aim to tackle these challenges and to remove obstacles to the development of a viable hydrogen industry by way of massive investments. France, for example, published a ‘National Strategy for the Development of Decarbonated Hydrogen’ in September 2020, with a budget of 7 billion euros for the ten-year period ending in 2030. This amount is equivalent to the investment announced by Portugal the same year, and slightly less than Germany’s 9 billion euro plan. It is also in line with the roadmaps already created by a number of countries, including the Netherlands, Korea, Japan and the United States, among others.
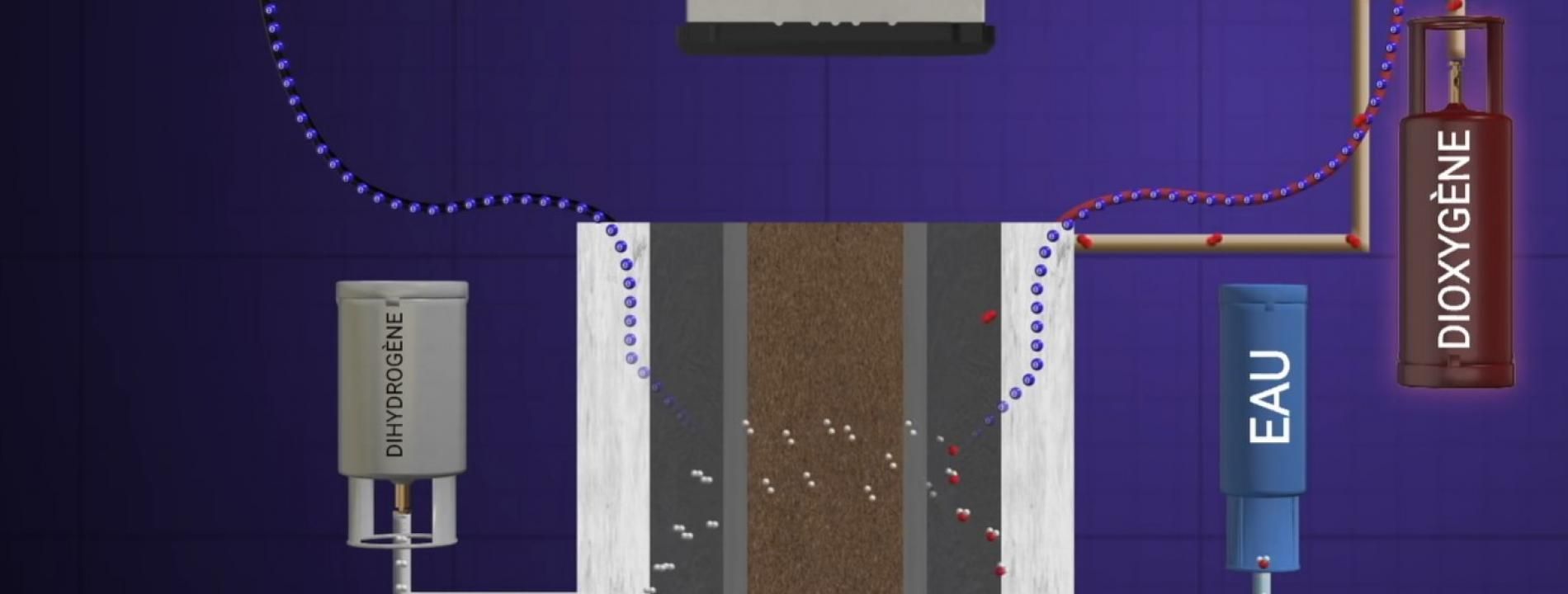
Clean… but locally
Hydrogen is considered a zero carbon energy source insofar as its use in a fuel cell—like the one powering this engine – emits only water, according to the formula 2 H2 + O2 → 2 H2O + electricity. But the way hydrogen is produced has to be taken into account too. If it is the product of fossil fuels (as is generally the case today), harmful emissions (carbon monoxide, nitrogen oxides) or greenhouse gases will be generated prior to its use as an energy source. This means that emissions are merely transferred from the exhaust pipe to the factory chimney. While this still could be of interest in reducing the noxious emissions that are suffocating cities, it does nothing to offset climate change.

Green transportation… but expensive
Hydrogen is seen as a promising solution for greener forms of mobility. It offers a better range than electric batteries and shorter recharging times. In practice, however, the technology seems best suited for trains and maritime freight, as the constraints linked to safety and on-board hydrogen storage are not as strong as they are for individual modes of transit. And the additional cost is not as marked.
Energetic… but dangerous
Hydrogen is one of the most energetic elements known… but it is also one of the most highly flammable.
The main interest of hydrogen is that it is very energy dense. It has the highest mass-based energy density of all elements, almost two and a half times higher than that of natural gas. As a result the combustion of one kilogram of hydrogen releases about three times more energy than the same amount of gasoline (with the advantage of producing only water). The flip side of the coin is that this colourless and odourless gas can be very dangerous. For hydrogen is also one of the most flammable substances, as was demonstrated for all to see when the Hindenburg transatlantic airship burst into flames in 1937 or when the American shuttle Challenger disintegrated in 1986, due to a leak in its hydrogen tank just after take-off. These events left a lasting impression on the public and the image of hydrogen as a dangerous substance took hold in people’s minds. However, the conditions under which hydrogen presents a risk of detonation are limited. Due to its high buoyancy and diffusivity (four times faster than natural gas), it tends to disperse rapidly in the event of leakage, instead of concentrating. This limits the risk of spontaneous detonation.
Light… but difficult to transport
Lightness would seem to be an advantage for transportation.
But in actual fact it results in strong constraints in terms of pressure and temperature. Hydrogen is the lightest atom: its nucleus has only one proton and no neutrons. And in its H2 (dihydrogen) form, it is the lightest of gases: twice as light as helium and eleven times as light as the air we breathe. On the down side, for the same mass, it is more voluminous than any other gas. This poses a problem of bulk for storage and transport. For example, an 11,000-litre tank would be needed to store 1 kg of hydrogen, which is the quantity required to travel 100 km by car. There are two options for resolving this problem. One is to cool the hydrogen to its liquefaction temperature of -252.87 °C, in which case, a 15-litre tank of liquid hydrogen would suffice to drive 100 km. But maintaining such a low temperature is a real challenge, which is why this solution is only being applied in very specific, demanding cases, such as for space rocket tanks. The solution practiced most often consists in compressing the gas (to under 350 to 700 bar pressure) to increase its density, and thereby reduce its volume. At 700 bars, the quantity needed to travel 100 km is contained in a volume of 25 litres. This still demands tanks that are both highly resistant and perfectly impermeable to prevent leakage: the hydrogen molecule is so small that it can pass through many materials, including metal, which it fragilizes and embrittles. Hence the use, for example, of tanks made of composite materials (for high strength) and lined with polymer resin (for impermeability).

Available at the pump… but only rarely
Recharging a fuel cell powered vehicle requires about 5 kilos of hydrogen at 350, 550 or 700 bar compression (depending on the vehicle), and takes only a few minutes. At 1 kg of hydrogen per 100 km, the current cost stands at 15 euros compared with about 10 euros for a gasoline-powered car. Moreover, the distribution network is still very limited. By the end of November 2020,* France had a network of only 41 hydrogen stations, many of which were privately owned and reserved for companies use. By way of comparison, there are 11,000 fuel service stations throughout the country.
* Source: Association française pour l’hydrogène et les piles à combustible (Afhypac)
Abundant… but not on Earth
Found in great quantities in the Universe, hydrogen in its gaseous form is barely present on Earth.
It is the paradox of hydrogen: it is at once abundant … and rare. Abundant because it is one of the very first atoms to appear in the Universe in the fractions of a second following the Big Bang 13.8 billion years ago, and it remains the most common chemical element by far, accounting for 74% of the total mass of the observable Universe. It is found in interstellar space, and it is the ‘fuel’ for galaxy formation; it is notably the main constituent of stars, such as our Sun. On Earth, on the other hand, hydrogen is scarce. At least in the gaseous form (the dihydrogen molecule: H2) that is used in fuel cells. Dihydrogen is present in the atmosphere, but in minute quantities: 0.000055% in volume, far behind nitrogen (78%), oxygen (21%), argon (0.93%) and CO2 (0.04%). It is so light that it escapes into space. This is why hydrogen, which is also extremely sensitive and reactive, is generally found on Earth only in combination with other atoms to form chemical compounds, such as water (H2O), or hydrocarbons, such as methane (CH4). A preliminary production step is therefore needed to extract the hydrogen from these different forms before it can be used.
Natural reserves… but unexploitable
We have known since the 1970s that there is hydrogen escaping from certain ocean ridges, but at such depths that its exploitation seems hardly viable. Recently, natural deposits of hydrogen have been identified in the Philippines, Turkey and Russia. But they are few in number, and the emissions are too weak to be exploited. The location of these natural sources are sometimes indicated by the presence of rounded depressions devoid of vegetation that can reach several kilometres in diameter: over time, hydrogen seepage into the soil causes destructuration, compaction and sterility.

A carrier of electricity… but hard to store
Hydrogen could serve to balance recurrent power grid fluctuations.
But the process is still a matter of debate. A primary fuel alternative for the transition to zero carbon mobility, hydrogen could also solve the problem of large-scale transient energy storage. The principle is rather simple, at least on paper: when the production of a power plant (nuclear, coal, wind or solar) exceeds demand on the grid, the surplus could be used to produce hydrogen by electrolysis of water. This hydrogen reserve could then supply electricity via fuel cells during peak consumption periods. The process would make it possible to compensate for fluctuations in the supply of intermittent renewable energies (solar, wind), which do not necessarily generate electricity when it is needed most. In practice, this requires low-cost, large-capacity electricity/hydrogen conversion facilities as well as bulk storage facilities. One option being considered involves injecting the hydrogen into underground salt caverns in structures at a depth of up to 2 km, with the salt forming a natural sealed barrier. This solution, already practiced for natural gas storage, tends to face resistance from local residents due to fear of leaks or explosions.

First hydrogen power plant… but not very powerful
This power plant, inaugurated in December 2019 in Le Lamentin, Martinique, is the first facility in the world to use the hydrogen generated by an oil refinery (in this case the SARA refinery to which it is attached) to produce electricity, which is then injected into the power grid. This so-called ‘grey’ hydrogen – a by-product of the refining process – is usually burned rather than recovered and put to use. The experimental plant is equipped with one of the largest fuel cells currently in operation in the world. However, its capacity is 1 megawatt (MW), very far from the minimum 900 MW of the reactors in France’s nuclear power plants.